Medicinal product safety
A probiotic which is registered as a medicinal product must comply with high standards. As opposed to dietary supplements or balanced diets, evidence of clinical efficacy must be submitted for defined medical indications and documents on efficacy and action mechanisms are required. In addition, the compliance with special product quality and safety criteria must be guaranteed (e. g. microbiological purity and defined viable cell counts as well as the non-pathogenicity and genetic stability of the therapeutically applied microorganism). 11
Mutaflor fulfills all modern-day safety requirements of a microbial medicinal product comprehensively and may therefore be applied even in high doses to patients of any age group and to pregnant and breast-feeding women as well.
E. coli stain Nissle 1917 is deposited in the German Collection of Microorganisms and Cell Cultures in Brunswick under the designation of E. coli DMS 6601 and it is the probably best investigated therapeutically applied E. coli strain worldwide. It non-pathogenicity has been elucidated down to the level of molecular genetics. 61; 62 The progress made in deciphering the genome of EcN has resulted in the development of a specific DNA-based PCR identification method, allowing a direct determination of Mutaflor in patient stools samples. 63 The genome 64 of EcN has been completely sequenced by now. The comparative sequence analysis indicates which DNA segments encode the known properties of the strain and defines where in the genome yet other genes responsible for hitherto unknown characteristics are localized.
E. coli strain Nissle 1917 is non-pathogenic
The comprehensive research results prove that E. coli strain Nissle 1917 in Mutaflor does not have any pathogenic properties. It produces neither hemolysin nor other toxins, displays no mannose-resistant hemagglutination; it is not serum-resistant, possesses no typical features of uropathogenicity and is not invasive. The pathogenicity features typical of pathogenic E. coli-bacteria do not exist in the EcN strain. The Central Committee for Biological Safety (ZKBS) therefore assigned EcN to risk group 1 (safe microorganisms).


Lane 1: DNA length standard
Lanes 2–7: EcN cultures
Unambiguous molecular biological identification
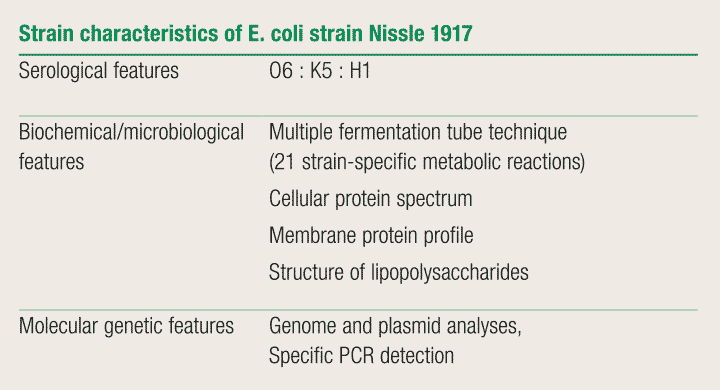
EcN can be identified by an analysis of its strain-specific metabolic reactions, its cell protein patterns or cell wall structures, by a differential molecular analysis of the bacterial DNA, or by means of strain-specific DNA probes and subsequent polymerase chain reaction (PCR). 63
E. coli strain Nissle 1917 does not possess any antibiotic resistance plasmids
What is very important nowadays: As an isolate of the pre-antibiotic era, E. coli strain Nissle 1917 does not possess any antibiotic-resistance plasmids. Furthermore, EcN is genetically stable and a poor recipient of foreign DNA in the form of antibiotic resistance and other promiscuous plasmids.
11) Schiemann M et al. 125 Jahre E. coli – Bedeutung in Forschung und Medizin. Alfred-Nissle-Gesellschaft (Hrsg.) Hagen, ISBN 3-9811198-4-3, 2010.
61) Grozdanov L et al. A single nucleotide exchange in the wzy gene is responsible for the semirough O6 lipopolysaccharide phenotype and serum sensitivity of Escherichia coli strain Nissle 1917. J Bacteriol 2002; 184: 5912–5925.
62) Grozdanov L et al. Analysis of the Genome Structure of the Nonpathogenic Probiotic Escherichia coli Strain Nissle 1917 J Bacteriol. 2004; 186: 5432–5441.
63) Blum-Oehler G et al. Development of strain-specific PCR reactions for the detection of the probiotic Escherichia coli strain Nissle 1917 in fecal samples. Res Microbiol 2003; 154: 59–66.
64) Reister M et al. Complete genome sequence of the Gram-negative probiotic Escherichia coli strain Nissle 1917. J Biotechnol 2014;187:106–107.
